Acheron Medical Legal Review (MLR) – A Product Overview
Medical Legal Review (MLR) has always been one of the most challenging and complex aspects of the marketing production process for Life Sciences companies.
Organizations need a fresh approach to MLR that enables a timely and efficient marketing workflow and allows for rapid process innovation.
Acheron Medical, Legal and Regulatory Compliance & Approval Solution provides a comprehensive platform to manage the MLR reviews and approvals, ensure regulatory compliance and improve the management of creative teams and assets.
It helps your organization to digitize, streamline and accelerate the MLR end-end process.

Acheron MLR serves target audiences such as CMOs, VP of Marketing, Customer Experience, and Customer Service, Brand Managers, Creative and Marketing Teams, Marketing Operations Teams, and Project Management Officers
-
Written by

-
Team AcheronMarketing
- July 5, 2021
- 3 mins read
Product Overview
Acheron MLR solution is built on OpenText Media Management (OTMM) and AppWorks platform to manage the life cycle of promotional material. It leverages the OTMM for the content management, versions management, metadata, search, and other media operations.
Acheron MLR solution leverages AppWorks for the lifecycle management of the content, external system integration and master data management.
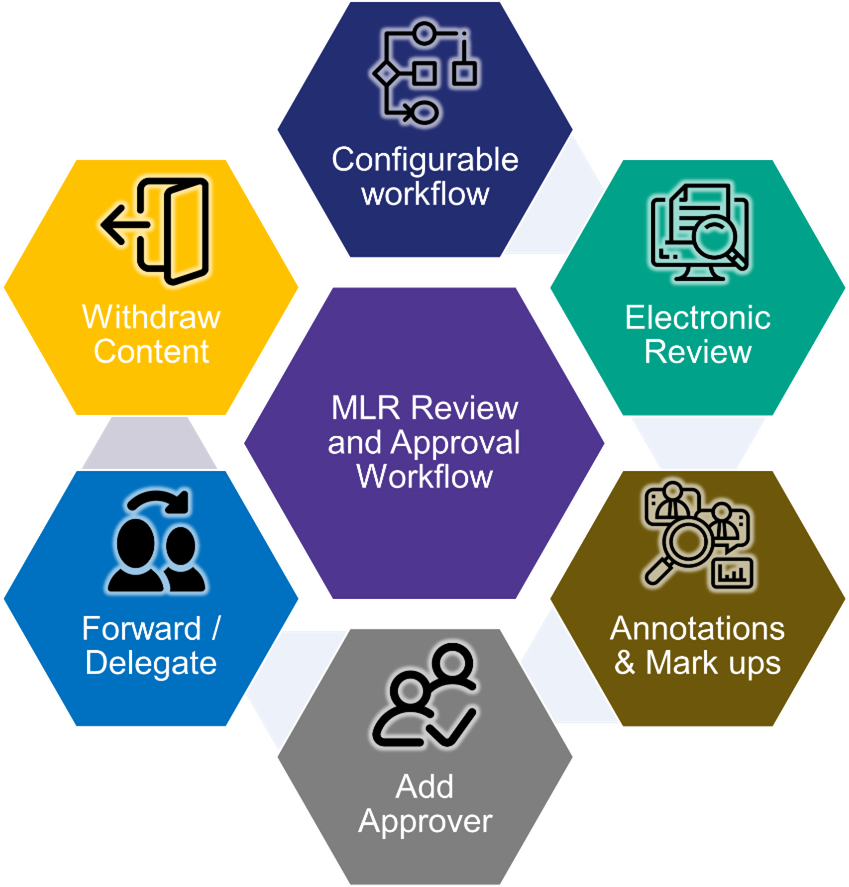
With Acheron MLR solution, the upstream creation, promotional/regulatory review, and downstream publishing/distribution happens in one system where asset management and compliant review happens in unison fashion. Acheron MLR solution replaces manual processes with system driven workflows, reviews, proactive notification, and digital collaboration.
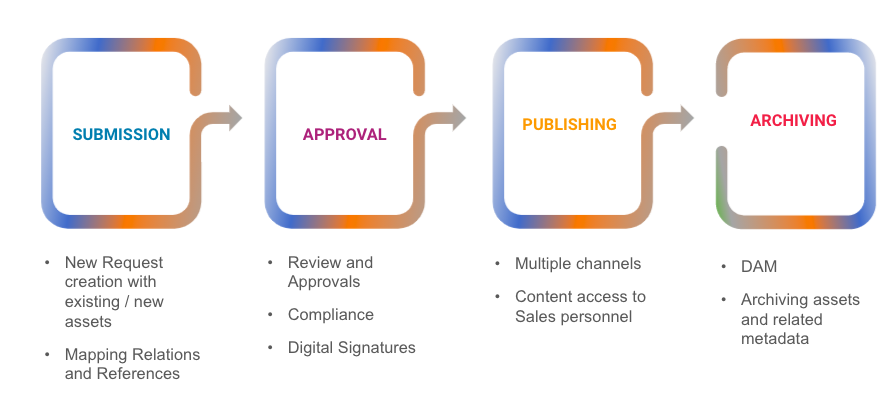
Acheron MLR solution, with unified experience of the Media Management and its workflows enables users to perform process related operations and asset related operations in one place.
Key Capabilities
- A single source for Planning, Tracking, Authoring and Publishing regulated content
- Solution for quicker Submissions with enhanced Collaboration and reporting
- Secured access and structural storage of content across the globe assisting rapid search
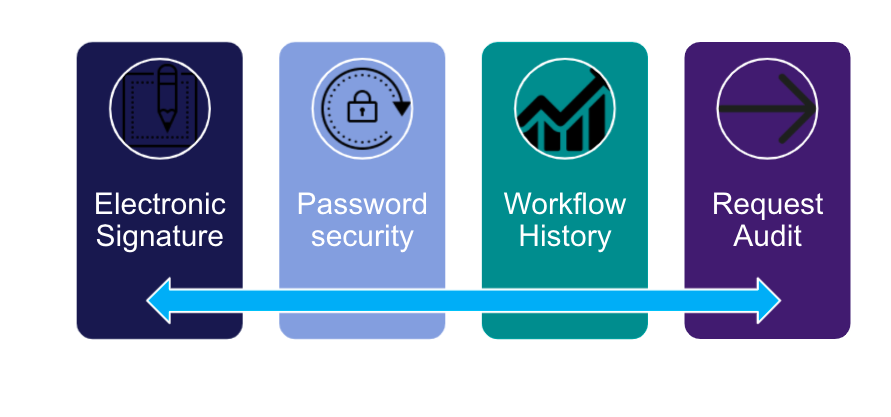
- Workflow and Audit tracking mechanisms along with notification capabilities
- Reference and Relationship management linking global contents
- Configurable workflows aligning to various market needs
- Fully integrated into OpenTextTM Media Management with various upstream and downstream channels
Value Proposition
- Stronger Regulatory Compliance
- “Point & Click” distribution and Automated withdrawal
- Improved Global brand consistency
- Multi-channel interaction and publishing
- Improved Efficiency and Team Productivity
- Stronger collaboration across the internal and external agencies
- Integrated metadata with external systems
More to explore
MongoDB Connector for Camunda 8- Part III
In the previous segment, we honed our data retrieval skills, allowing us to uncover valuable insights from our collections in MongoDB database. In this concluding part, let’s discuss the remaining operations like updating documents, deleting documents,
MongoDB Connector for Camunda 8- Part II
In the previous part, we laid the groundwork by creating collections and inserting documents into them. Now, it’s time to extract valuable insights from your data using MongoDB’s robust querying

GCP Cloud Spanner Connector for Camunda 8
Integrating Camunda with Google Cloud Spanner offers a powerful combination of workflow automation and a globally distributed, horizontally scalable database. Google Cloud Spanner provides strong consistency and high availability, making it suitable for

MongoDB Connector for Camunda 8- Part I
Integrating Camunda, a popular open-source workflow automation and business process management platform, with MongoDB, a NoSQL database, can offer powerful capabilities for managing and executing business processes while efficiently storing and retrieving

Google Cloud Storage Connector
Whether you’re saving photos, important documents, or sharing files on GCS – this GCS Connector makes managing your digital content more convenient for intended users. It’s like having a reliable assistant for your data tasks, making things

Acheron Newsletter Q1 FY23
A growth strategy is an organisation’s plan for achieving current and future objectives to realize its goals of

Software Testing – Third Eye View
Everyone knows what Software Testing is, but here are some challenges and how to overcome those challenges. The primary focus of a software tester would be to stay very close to theoretical stuff and still reach out to match the reality by understanding the

Our Solution to your Market Penetration Strategy.
Who doesn’t want their business to grow? In fact everyone like to see growth in business. This seems pretty true – Considering effective and successful growth means your business is experiencing a boost in revenue, brand awareness, brand

Acheron’s CHILI Publish connector for OpenText Media Management
The technological advances that have occurred in the Media Industry (print & digital) in the past decade has presented content creators with a wealth of new revenue streams. Increasingly, Media based industries around the globe are implementing

Acheron MLR Digital Publishing App – An Overview
Medical Legal Review has always been one of the most challenging and complex aspects of the marketing production process for Life Sciences companies. Life science company’s field force (including but not limited to AEs, MSLs, Field Marketing etc..) has not

Acheron Medical Legal Review (MLR) – A Product Overview
Medical Legal Review (MLR) has always been one of the most challenging and complex aspects of the marketing production process for Life Sciences companies. Organizations need a fresh approach to MLR that enables a timely and efficient marketing workflow and

Acheron Media Project Management (MPM) – A Product Overview
Acheron MPM is a comprehensive solution to empower the marketing teams and project manager to manage all their creative workflow in one place, from inception to consumption. It automates your digital media life cycle and delivers a holistic approach to

4 and a half reasons Standard Project Management applications fail media projects
I know that triggers a curiosity in your mind – what are these four and a half reasons? Well, thank you for taking your time to read through these findings that I could build over the past one and a half years of my exposure to this domain. You

Acheron Media Project Management (MPM) – An Executive Brief
One of the few challenges marketing project managers face in their routine is to keep track of tasks and their current status. This could be due to the complex workflows involved in their projects and having to work with a multitude of tasks that are