Acheron MLR Digital Publishing App – An Overview
Medical Legal Review has always been one of the most challenging and complex aspects of the marketing production process for Life Sciences companies.
Life science company’s field force (including but not limited to AEs, MSLs, Field Marketing etc..) has not been given access to the published content in an easy and intuitive way.
Field force is forced to use old school client-server-based solution with thick client to access the published content.
Working with outdated content puts the team under tremendous pressure by having un-productive sales calls, printing the content, long sales cycle, etc.
For these companies, access to the published content at the right time in an easy intuitive manner would accelerate the sales, increase their productivity, and avoid any FDA penalties.

Acheron MLR serves target audiences such as CMOs, VP of Marketing, Customer Experience, and Customer Service, Brand Managers, Creative and Marketing Teams, Marketing Operations Teams, and Project Management Officers
-
Written by
-
Team AcheronMarketing
- July 5, 2021
- 4 mins read
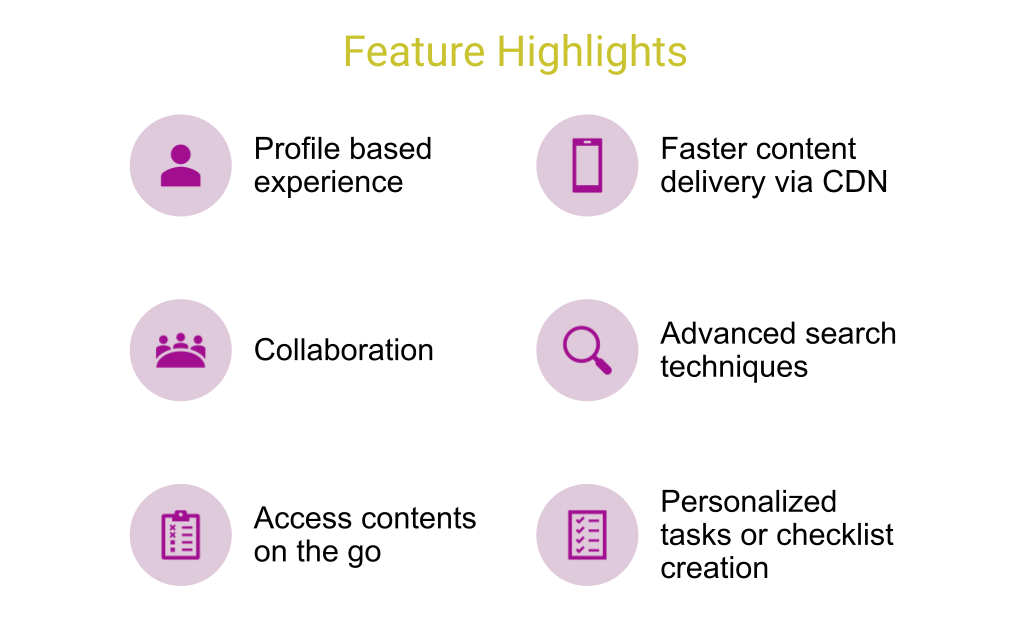
Product Overview
MLR Publishing App provides unified solution for publishing that significantly speeds the downstream publishing and regulated content submission delivery.
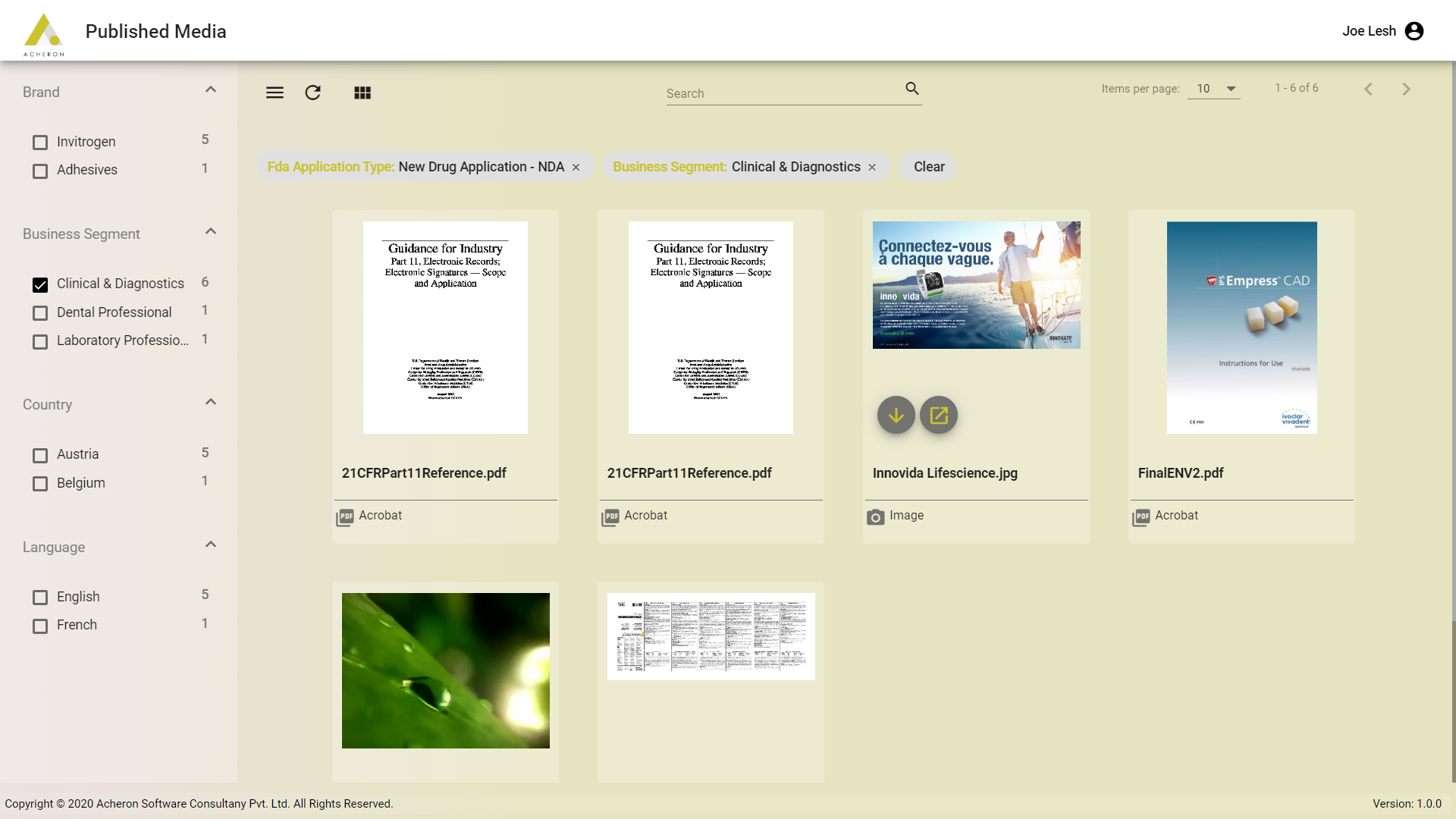
With content managed in OpenText Media Management and MLR regulated approval process managed and governed in MLR solution, the Publishing App provides a unified experience for the field force to access and consume the published content. The publishing App can be configured to show the subset and filtered content from OTMM.
The filter can be around the approval status, brand and any other metadata. The concept is called as “Profiling.” This allows the Life Science companies to launch and configure multiple instances of the publishing App.
Profiling allows to perform such operations. These instances of the app can be deployed to meet the multiple regions, products, compliance, and regulatory requirements. The solution also moves referenced-document hyperlinking upstream during the creation phase and away from the final publishing phase.
With the continuous delivery and continuous integration process the solution performs validation and reference checking before the content is delivered in the app. The app offers greater publishing automation, transparency and velocity.
Improved Productivity
Given AEs, MSLs and Marketing team access to the published content, the team is prepared to respond fast and easy, handle customer queries and physician are appreciative of the more productive discussions.
It provides direct access to the correct version of the content without performing any manual checks and content duplication.
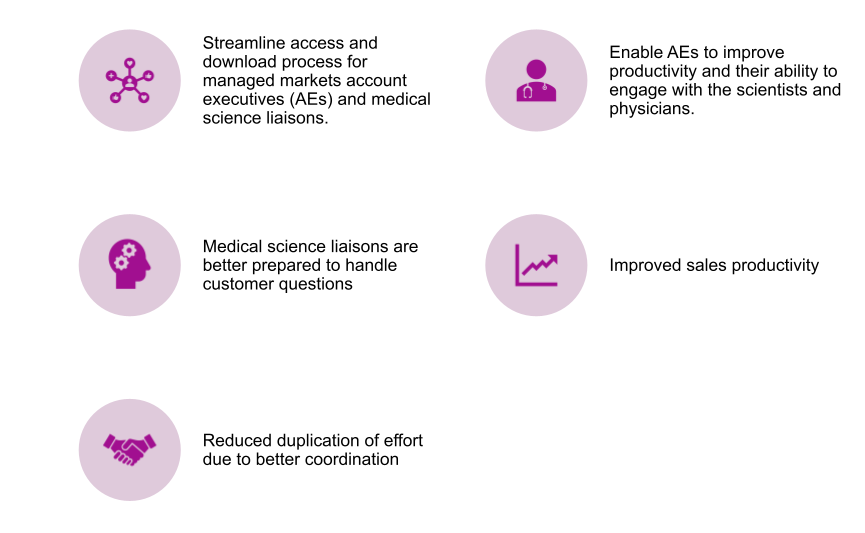
Shorter Lead time
With the publishing process, steps are completed earlier and real-time published content to the field force, the app rapidly reduces the total end-end lead time.
Global Alignment
Establish greater control over the distribution and usage of documents that affiliates submit to local regulatory authorities.
Single global, authoritative source
Best practice for information governance would dictate that there be one authoritative system of record for business documents and consequently, one version of the truth.
MLR solution with its preconfigured integrations to the most common content repositories resolve the issue of information silos, without disrupting the existing infrastructure or the workflows used by different departments and teams.
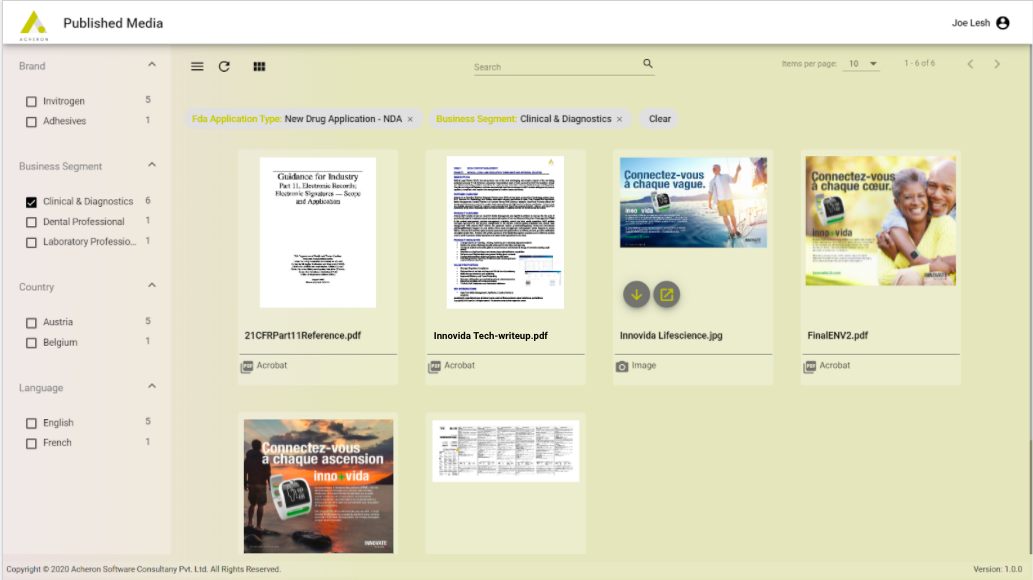
Balance Security and Access
Solution provides dynamic access control based on business rules enabling the right users to get the appropriate assets and asset security managed at various stages in the lifecycle.
Digital publishing app provides access to the content based on the RBAC policies.
More to explore
MongoDB Connector for Camunda 8- Part III
In the previous segment, we honed our data retrieval skills, allowing us to uncover valuable insights from our collections in MongoDB database. In this concluding part, let’s discuss the remaining operations like updating documents, deleting documents,
MongoDB Connector for Camunda 8- Part II
In the previous part, we laid the groundwork by creating collections and inserting documents into them. Now, it’s time to extract valuable insights from your data using MongoDB’s robust querying

GCP Cloud Spanner Connector for Camunda 8
Integrating Camunda with Google Cloud Spanner offers a powerful combination of workflow automation and a globally distributed, horizontally scalable database. Google Cloud Spanner provides strong consistency and high availability, making it suitable for

MongoDB Connector for Camunda 8- Part I
Integrating Camunda, a popular open-source workflow automation and business process management platform, with MongoDB, a NoSQL database, can offer powerful capabilities for managing and executing business processes while efficiently storing and retrieving

Google Cloud Storage Connector
Whether you’re saving photos, important documents, or sharing files on GCS – this GCS Connector makes managing your digital content more convenient for intended users. It’s like having a reliable assistant for your data tasks, making things

Acheron Newsletter Q1 FY23
A growth strategy is an organisation’s plan for achieving current and future objectives to realize its goals of

Software Testing – Third Eye View
Everyone knows what Software Testing is, but here are some challenges and how to overcome those challenges. The primary focus of a software tester would be to stay very close to theoretical stuff and still reach out to match the reality by understanding the

Our Solution to your Market Penetration Strategy.
Who doesn’t want their business to grow? In fact everyone like to see growth in business. This seems pretty true – Considering effective and successful growth means your business is experiencing a boost in revenue, brand awareness, brand

Acheron’s CHILI Publish connector for OpenText Media Management
The technological advances that have occurred in the Media Industry (print & digital) in the past decade has presented content creators with a wealth of new revenue streams. Increasingly, Media based industries around the globe are implementing

Acheron MLR Digital Publishing App – An Overview
Medical Legal Review has always been one of the most challenging and complex aspects of the marketing production process for Life Sciences companies. Life science company’s field force (including but not limited to AEs, MSLs, Field Marketing etc..) has not

Acheron Medical Legal Review (MLR) – A Product Overview
Medical Legal Review (MLR) has always been one of the most challenging and complex aspects of the marketing production process for Life Sciences companies. Organizations need a fresh approach to MLR that enables a timely and efficient marketing workflow and

Acheron Media Project Management (MPM) – A Product Overview
Acheron MPM is a comprehensive solution to empower the marketing teams and project manager to manage all their creative workflow in one place, from inception to consumption. It automates your digital media life cycle and delivers a holistic approach to

4 and a half reasons Standard Project Management applications fail media projects
I know that triggers a curiosity in your mind – what are these four and a half reasons? Well, thank you for taking your time to read through these findings that I could build over the past one and a half years of my exposure to this domain. You

Acheron Media Project Management (MPM) – An Executive Brief
One of the few challenges marketing project managers face in their routine is to keep track of tasks and their current status. This could be due to the complex workflows involved in their projects and having to work with a multitude of tasks that are